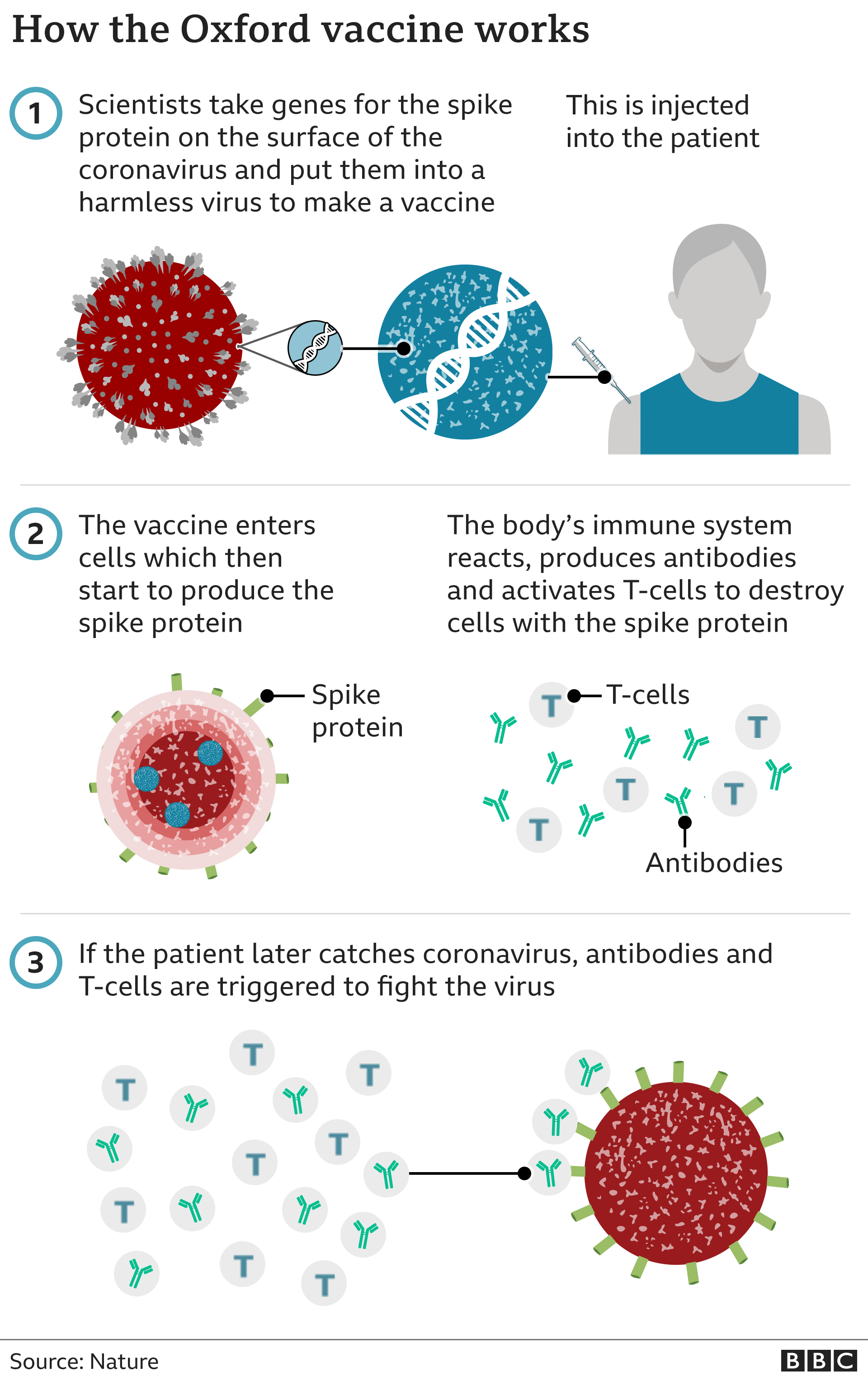
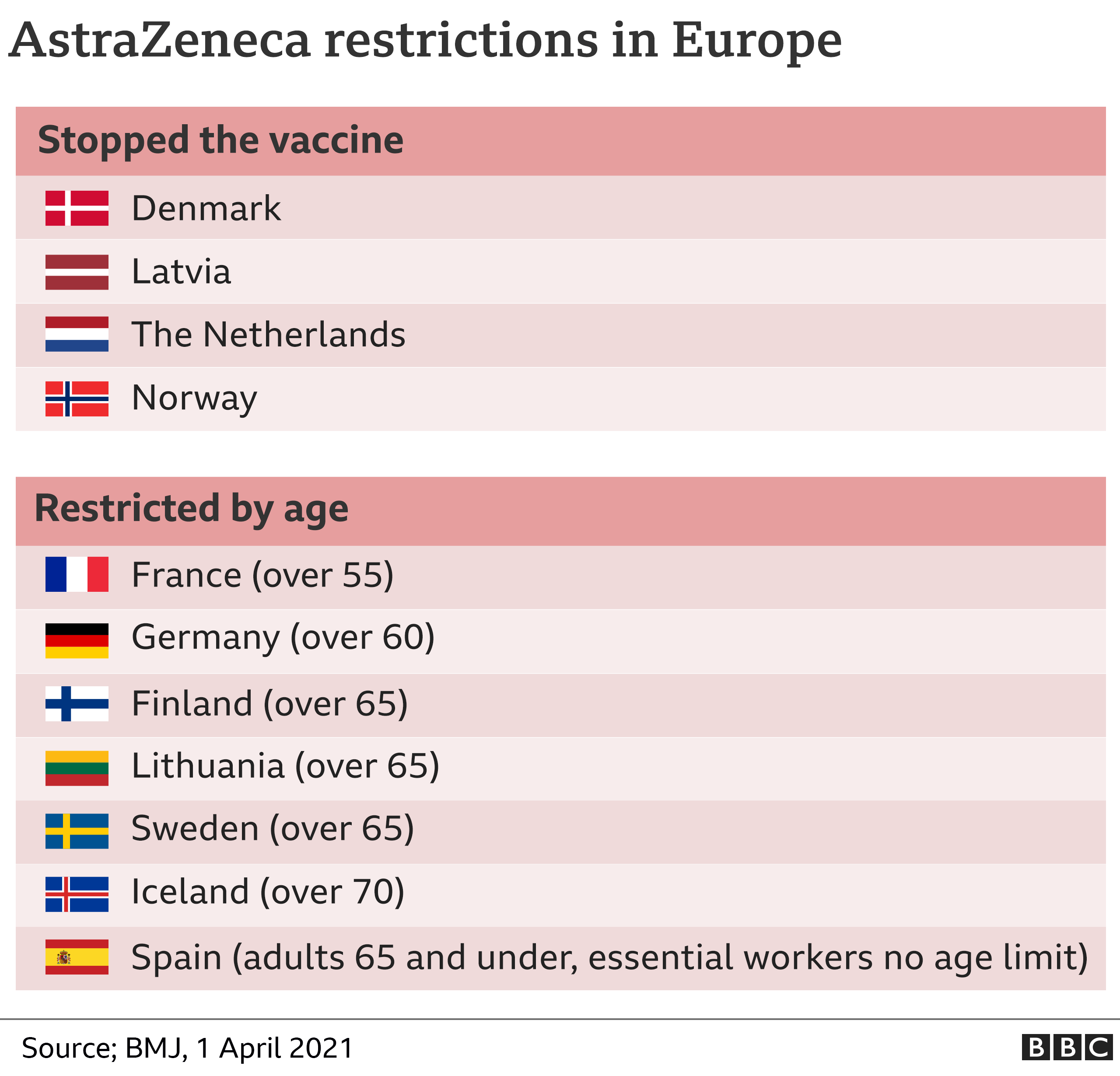
Under-30s in the UK are to be offered an alternative Covid vaccine to the AstraZeneca jab due to the evidence linking it to rare blood clots.
The recommendation comes after a review by the UK drugs regulator found that by the end of March 79 people had suffered rare blood clots after vaccination - 19 of whom had died.
The regulator said this was not proof the jab had caused the clots.
But it said the link was getting firmer.
The review by the Medicines and Healthcare products Regulatory Agency (MHRA) found:
- The 79 cases and 19 deaths occurred after 20 million doses were administered - giving a risk of about four in one million of developing a blood clot, and one in a million of dying
- Nearly two-thirds of the cases of rare clots were seen in women
- The people who died were aged between 18 and 79, with three of them aged under 30
- All the recorded cases occurred after the first dose, although the lower number of second doses meant it was not possible to draw any conclusions from this
Meanwhile, the EU's medicines regulator says unusual blood clots should be listed as a possible very rare side effect of the AstraZeneca jab, but that the benefits outweighed the risks. Some European countries have restricted the vaccine's use.
The World Health Organization said the link between the vaccine and blood clots was "plausible" but not confirmed, adding that the clotting incidents were "very rare" among nearly 200 million people who have received the jab worldwide.

Analysis: Should we be worried about a one-in-a-million risk?
No treatment or vaccine is risk free. The key question is whether it does more good than harm.
Wednesday's update once again demonstrates the AstraZeneca vaccine does - even if you assume it's causing these clots, which has not been proven yet.
The risk of dying from one of them following vaccination is incredibly small - about one in a million.
By contrast, Covid kills one in eight people who are infected over the age of 75, and one in 1,000 infected in their 40s among those who develop symptoms.
It is less clear cut for those under 30, who are much less likely to die of Covid - although the AstraZeneca vaccine still presents more benefit than risk.
However, other vaccines may be an even better bet.
The risk might look worrying, but it is actually very low, and usually we don't think about things just in terms of risk.
For example, travelling 250 miles in a car also carries with it a one-in-a-million chance of dying in an accident. How many think about that when they get behind the wheel?

Health Secretary Matt Hancock said the UK's review confirmed that the Oxford-AstraZeneca jab is "safe, effective and the benefits far outweigh the risks for the vast majority of adults".
Prime Minister Boris Johnson said the AstraZeneca vaccine had "already saved thousands of lives" and the new advice should ensure people of all ages "continue to have full confidence in vaccines".
Labour leader Sir Keir Starmer also urged people to "trust in our doctors and scientists" and said he was looking forward to receiving his second AstraZeneca dose.
The UK reported a further 45 deaths from Covid-19 and another 2,763 confirmed cases on Wednesday. Vaccinations remained low after the Easter weekend, with 186,793 second doses administered and 85,227 first doses.
June Raine, chief executive of the MHRA, said the side-effects of the AstraZeneca vaccine were "extremely rare" - and more work was going to identify if the vaccine was definitely causing the clots.
"The balance of benefits and known risks is still very favourable for the majority of people," she said.
But she said for younger age groups it was more "finely balanced".
She added: "The public's safety is at the forefront of our minds."
Dr Raine said there was a "reasonably plausible" link between the vaccine and the blood clots, although AstraZeneca has said its studies have found no causal connection.
The review prompted the UK government's vaccine advisory group, the JCVI, to recommend that people aged 18 to 29 be offered an alternative vaccine where available.
Professor Lim Wei Shen, of the JCVI, said the move was being made "out of the utmost caution rather than because we have any serious safety concerns".

Professor Sir Munir Pirmohamed, chairman of the Commission on Human Medicines, said the risks had to be weighed against the consequences of Covid-19, which also causes clotting.
He said 7.8% of coronavirus patients suffer blood clots on the lungs, while 11.2% will suffer deep vein thrombosis (DVT) in the legs.
People who have had their first dose of the AstraZeneca vaccine should still get their second dose, the MHRA said. Only those who suffered one of these rare blood clots after the first dose should not get vaccinated, it added.
Pregnant women and people with blood disorders that leave them at risk of clotting should discuss the benefits and risks of vaccination with their doctor before going for a jab.
Anyone who suffers symptoms such as a persistent headache, blurred vision or confusion for four days or more after vaccination or who experience unusual skin bruising, shortness of breath or chest pain are being asked to seek medical advice.
England's deputy chief medical officer, Prof Jonathan Van-Tam, described the move as a "course correction" - and said it was normal in medicine to change preferences in this way.
He also said the impact on the government's promise to offer all adults a jab by the end of July should be "zero or negligible" as long as the expected supplies of the Pfizer and Moderna vaccines - the other two Covid vaccines in use in the UK - arrived as expected in the coming months.

- LOOK-UP TOOL: How many cases in your area?
- LOCKDOWN RULES: What are they and when will they end?
- YOUR QUESTIONS: We answer your queries
- GLOBAL SPREAD: How many worldwide cases are there?


- YOUR MENTAL HEALTH TOOLKIT: Tips and tools to look after your mental health
- WHO KILLED EMMA CALDWELL?: 16 years later her murder is still unsolved...

Are you under 30 and waiting to be vaccinated? Share your views and experiences by emailing haveyoursay@bbc.co.uk.
Please include a contact number if you are willing to speak to a BBC journalist. You can also get in touch in the following ways:
- WhatsApp: +44 7756 165803
- Tweet: @BBC_HaveYourSay
- Upload pictures or video
- Please read our terms & conditions and privacy policy
If you are reading this page and can't see the form you will need to visit the mobile version of the BBC website to submit your question or comment or you can email us at HaveYourSay@bbc.co.uk. Please include your name, age and location with any submission.
https://news.google.com/__i/rss/rd/articles/CBMiKmh0dHBzOi8vd3d3LmJiYy5jby51ay9uZXdzL2hlYWx0aC01NjY2NTUxN9IBLmh0dHBzOi8vd3d3LmJiYy5jby51ay9uZXdzL2FtcC9oZWFsdGgtNTY2NjU1MTc?oc=5
2021-04-07 17:26:21Z
52781491296352