The second dose of the coronavirus vaccines will be given later than originally planned, experts have said.
Live COVID updates from UK and around world
The move is to ensure more people are given a first dose to help fight the UK's rising coronavirus infection rate, with the second dose administered up to three months later.
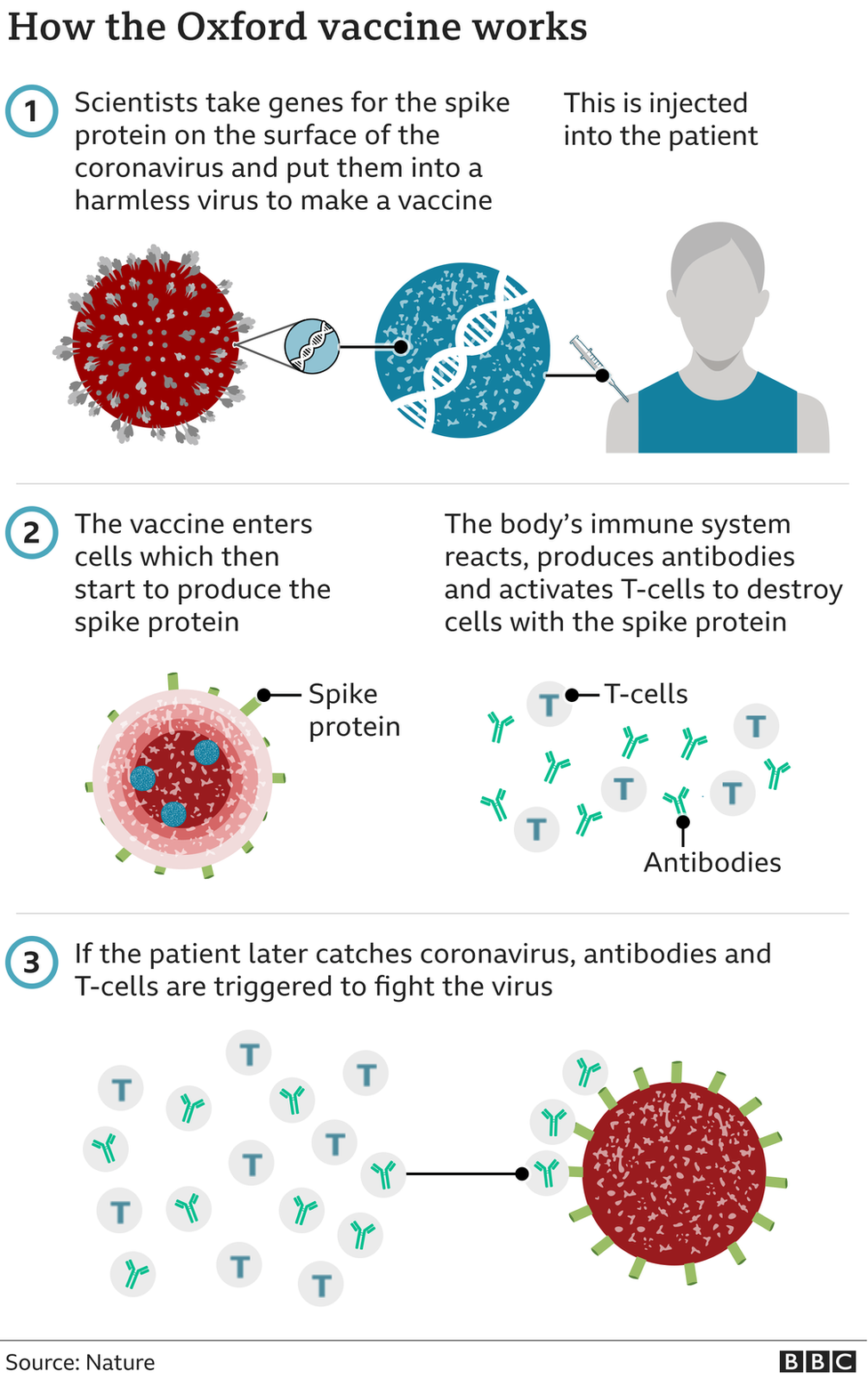
Speaking at a briefing at Downing Street, Professor Wei Shen Lim, chair of the Joint Committee of Vaccinations and Immunisations, said the "immediate urgency" was for the rapid rollout of the new Oxford/AstraZeneca vaccine and to ensure high levels of uptake.
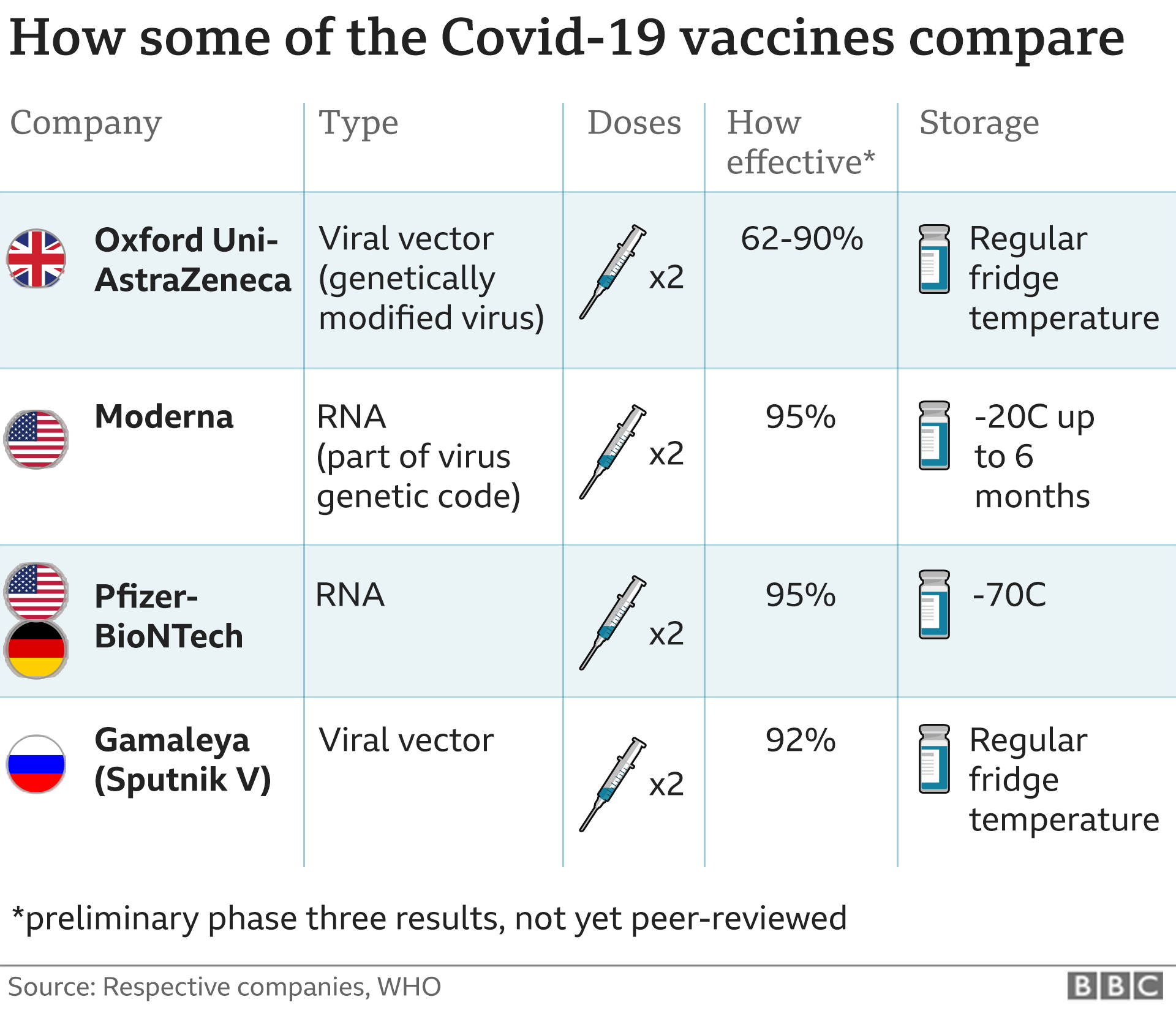
He said: "We recommend delivery of the first vaccine should be prioritised for both the Pfizer/BioNTech and the Oxford/AstraZeneca vaccine.
"This will allow the greatest number of people to receive the vaccine in the shortest possible time - and that will protect the greatest number of lives."
But Professor Sir Munir Pirmohamed, chair of the Commission on Human Medicine Expert Working Group which looked into the efficacy and safety of the Oxford vaccine warned it did not take effect until after three weeks, so safety measures should be followed in the interim.
A first dose of the jab gives around 70% effectiveness from three weeks after immunisation.
However, he said it was 80% effective when there was a three-month interval between the first and second doses.
He added: "We examined the half-dose regime but we felt that the results were not borne out by the full analysis."
The Oxford vaccine was approved for use in the UK earlier by the Medicines and Healthcare products Regulatory Agency (MHRA).
The first doses of the Oxford jab are due to be given on Monday amid rising coronavirus cases in the UK.
The panel also outlined new advice for dosage of the first approved vaccine - the Pfizer/BioNTech vaccine.
Previous guidance had suggested the second dose could be taken up to 21 days after the first, but today that changed to at least 21 days after the initial vaccine.
And it also announced changes to the advice for those with allergies and for those pregnant or breastfeeding women.
Dr Raine, chief executive of the MHRA, said previous advice had not recommended its use by pregnant and breastfeeding women due to "an initial lack of evidence on a precautionary basis".
But she told a briefing: "Now that we have reviewed further data that has become available, the Commission on Human Medicines has advised that the vaccine can be considered for use in pregnancy when the potential benefits outweigh the risks, following an individual discussion with every woman.
"And as the COVID-19 vaccine AstraZeneca is the same, women should always be discussing benefits and risks of having the vaccine with their health professional, reaching a decision together based on individual circumstances, and women who are breastfeeding can now also be given the vaccine, subject to that individual discussion."
On the issue of those with allergies, the advice also changed.
Dr Raine said growing evidence from a pool of at least 800,000 people in the UK and probably 1.5 million people in the US who have had the vaccine, had "raised no additional concerns".
This, she continued, "gives us further assurance that the risk of anaphylaxis can be managed through standard clinical guidance and an observation period following vaccination of at least 15 minutes.
"And so the Commission on Human Medicines has now advised that anyone with allergy to food or other medicine or vaccine can have the Pfizer/BioNTech vaccine.
"Of course, anyone with a history of allergic reaction to this vaccine, or its ingredients, should not."
https://news.google.com/__i/rss/rd/articles/CBMidGh0dHBzOi8vbmV3cy5za3kuY29tL3N0b3J5L2NvdmlkLTE5LXNlY29uZC1kb3NlLW9mLWNvdmlkLXZhY2NpbmVzLXRvLWJlLWdpdmVuLWxhdGVyLWFmdGVyLWd1aWRlbGluZXMtY2hhbmdlLTEyMTc1NDU20gF4aHR0cHM6Ly9uZXdzLnNreS5jb20vc3RvcnkvYW1wL2NvdmlkLTE5LXNlY29uZC1kb3NlLW9mLWNvdmlkLXZhY2NpbmVzLXRvLWJlLWdpdmVuLWxhdGVyLWFmdGVyLWd1aWRlbGluZXMtY2hhbmdlLTEyMTc1NDU2?oc=5
2020-12-30 13:30:00Z
52781275240124